ENT Institute Offering Robotic-Assisted Techniques for Some Cochlear Implantations
July 15, 2025
Innovations in Ear, Nose & Throat | Summer 2025
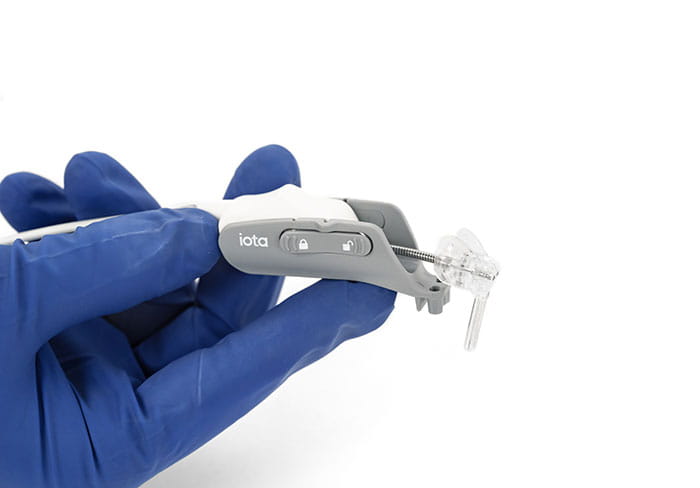
Surgeons within the University Hospitals Ear, Nose & Throat Institute are utilizing the first-in-class iotaSOFT® robotic insertion system for patients receiving Advanced Bionics cochlear implants (CI). Offered by iotaMOTION, the precision-driven robotic technology supports optimal cochlear placement and standardizes insertion speed, control and angle.
 Sarah Mowry, MD
Sarah Mowry, MD“For the past 15 to 20 years, we have been implanting folks with cochlear implants whose residual low-frequency hearing enhances their ability to detect background noise, appreciate music or perceive a sound’s location,” says Sarah Mowry, MD, Vice Chair for Education and the Cliff A. Megerian Chair for Excellence in Otolaryngology at University Hospitals Cleveland Medical Center and a professor at Case Western Reserve University School of Medicine. “There is merit to the idea that iotaSOFT’s robotic precision will enable surgeons to minimize intra-cochlear trauma and better protect remaining natural hearing.”
Authorized by the U.S. Food and Drug Administration, iotaSOFT was developed by a pair of otolaryngologists at the University of Iowa and represents a significant breakthrough in CI surgery. The robotic-assisted electrode array insertion device was engineered to maintain consistency throughout cochlear implantation, especially in complex anatomies or revision surgeries.
Early clinical evaluations show promising outcomes, when compared to manual insertion (per iotaMOTION):
- Improved insertion consistency
- 51 percent reduction in maximum insertion force
- 78 percent reduction in insertion force variation
Along with Dr. Mowry, Alejandro Rivas, MD, Maroun Semaan, MD, and Cameron Wick, MD, are certified to offer iotaSOFT to their patients.
“The innovative technique removes some of the human variability between providers and standardizes the amount of movement within the cochlea,” Dr. Mowry says. “The goal is to preserve both structure and function while optimizing the hearing outcomes that cochlear implants offer.”
Dr. Mowry’s first patient to benefit from the robotic surgery is doing very well. “This individual had a small degree of residual hearing and has significantly improved her speech recognition scores since her cochlear implant was activated,” she says. “The technique went smoothly and did not measurably add to operative time.”
The single-use iotaSOFT insertion system seamlessly integrates with standard CI surgical workflows. “The micro-robot is about the length of your thumb, so it is quite small in terms of its footprint,” Dr. Mowry says. “We attach it to the skull and thread the cochlear implant to insert into the cochlea.”
A reusable console with foot pedals enables the surgeon to control iotaSOFT, which employs a linear actuator to insert the electrode array at precisely controlled velocities. Moving at a range within sub-millimeters per second, the system reduces the risk of sudden movements or unintended force and enables standardization among operators.
Advancing National Excellence in Hearing Preservation
University Hospitals Cochlear Implant Center of Excellence+ is among the top 10 programs in the nation for the total number of CIs performed each year. The center continues to offer an unprecedented level of expertise and innovation in auditory research and hearing preservation.
University Hospitals is actively involved in clinical trials of drug-eluting cochlear implants, specifically investigating dexamethasone-eluting electrodes to reduce inflammation and improve hearing outcomes. The trials are part of a broader effort to improve CI technology and expand its benefits to a wider range of patients.
Working with research audiologist Viral Tejani, AuD, PhD, the team is also developing projects with Advanced Bionics to monitor hearing intraoperatively.
“We hope that utilizing the iotaSOFT robotic technology paired with intraoperative hearing monitoring will take our program to new levels of hearing preservation,” Dr. Mowry says. “As one of the larger volume cochlear implant centers across the country, we will continue conducting novel research for our patients, including those with significant residual low-frequency hearing.”
For more information, contact Dr. Mowry at Sarah.Mowry@UHhospitals.org.
Contributing Expert:
Sarah Mowry, MD
Vice Chair for Education
Cliff A. Megerian Chair for Excellence in Otolaryngology
Department of Otolaryngology
University Hospitals Cleveland Medical Center
Professor
Case Western Reserve University School of Medicine


